Ph.D. student in chemistry
Tohoku University
Japan
Even the high school/senior high school students are acquainted with the “Bohr model” which says electrons are "particles" that revolve around the nucleus in orbits and are quantized so that they can show absorption and emission phenomena.
It is a primitive model of the hydrogen atom though it is verified later after the introduction of the quantum mechanical concept.
 This model strongly supports the existence of the “Covalent Bond Theory [CBT]” and the “Valence Shell Electron Pair Repulsion theory [VSEPR]” of bonding between the atoms.
This model strongly supports the existence of the “Covalent Bond Theory [CBT]” and the “Valence Shell Electron Pair Repulsion theory [VSEPR]” of bonding between the atoms.The “Quantum model” on the other hand, says that electrons are not particles, but have wavelike characteristics just like photons and their wave length can be explained by the “de Broglie's equation”.
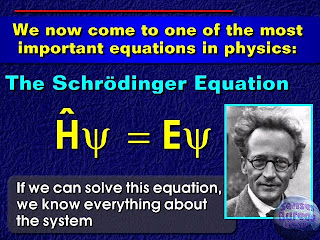
 In order to calculate the various properties of the electrons, Quantum model developed a famous equation by considering the “de Broglie concept” which is named as a Schrödinger’s equations and appeared as a stepping stone of the Quantum chemistry / Physics. I am sure that most of the chemistry undergrad./ grad. students are encountered with these two forms of Schrödinger equations:
In order to calculate the various properties of the electrons, Quantum model developed a famous equation by considering the “de Broglie concept” which is named as a Schrödinger’s equations and appeared as a stepping stone of the Quantum chemistry / Physics. I am sure that most of the chemistry undergrad./ grad. students are encountered with these two forms of Schrödinger equations: Ĥ ψ = E ψ & Ĥ ψ = iħ (δ/ δt)ψ (time independent and time dependent respectively). These equations model the atoms and molecules with mathematics.
It is well-known for the physical chemists that the quantum n-body problem [many electrons atoms or molecules, beyond Helium] cannot be solved analytically. However, the case for Hydrogen molecular ion [H2+ = 1 electron system] is relatively easy and the results normally agree with the information obtained by the chemical experiments. By considering such agreements, the necessity of the Quantum chemistry arises in order to verify and explore the chemistry of the micro particles which act as a foundation of the matter. The following movie has highlighted about the “Quantum chemistry in action” which explains the equilibrium process between ionized water and hydroxyl radical [OH.] plus hydronium ion [H3O+].
The detailed applications and the general way of simplifying Schrödinger equations will be posted on the days to come. Interested fellows are suggested to visit the site time to time.
---------------------To be continued-----------------------








No comments:
Post a Comment